
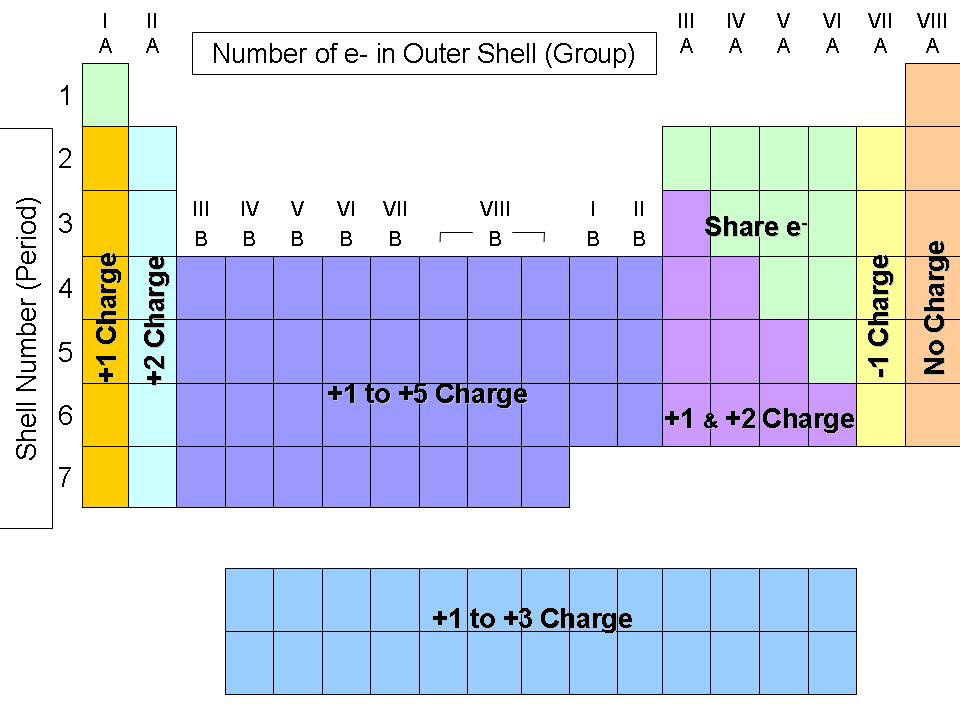
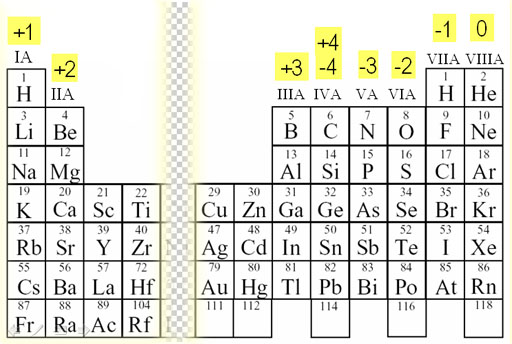
On the Periodic Table metals (found on the left of the table) will be. " So an element has a specific valence, depending on its group (e.g., C, 4 or Xe, 0), but may have multiple values for valency, such as in $\ce$. To find the ionic charge of an element youll need to consult your Periodic Table. Cations and anions When a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. This is a color-coded table that organizes all the known elements by atomic structure.

It's not simply the column that determines the ionic charge of an element, but also other factors, such as row and with what other elements it's combined.Įpediaa states, "valence refers to the ability of an atom to be combined with another atom whereas valency refers to the maximum number of electrons that an atom can lose or gain in order to stabilize itself. The resulting charged species is called an ion.


 0 kommentar(er)
0 kommentar(er)
